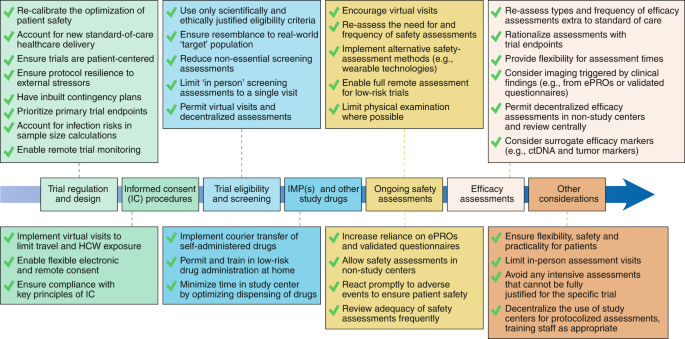
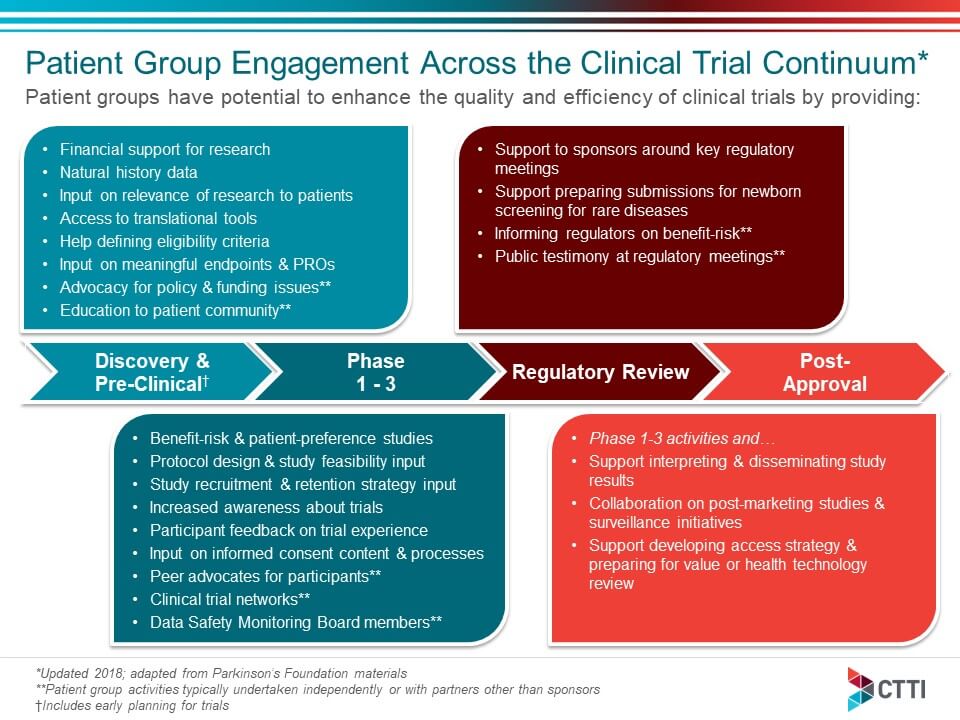
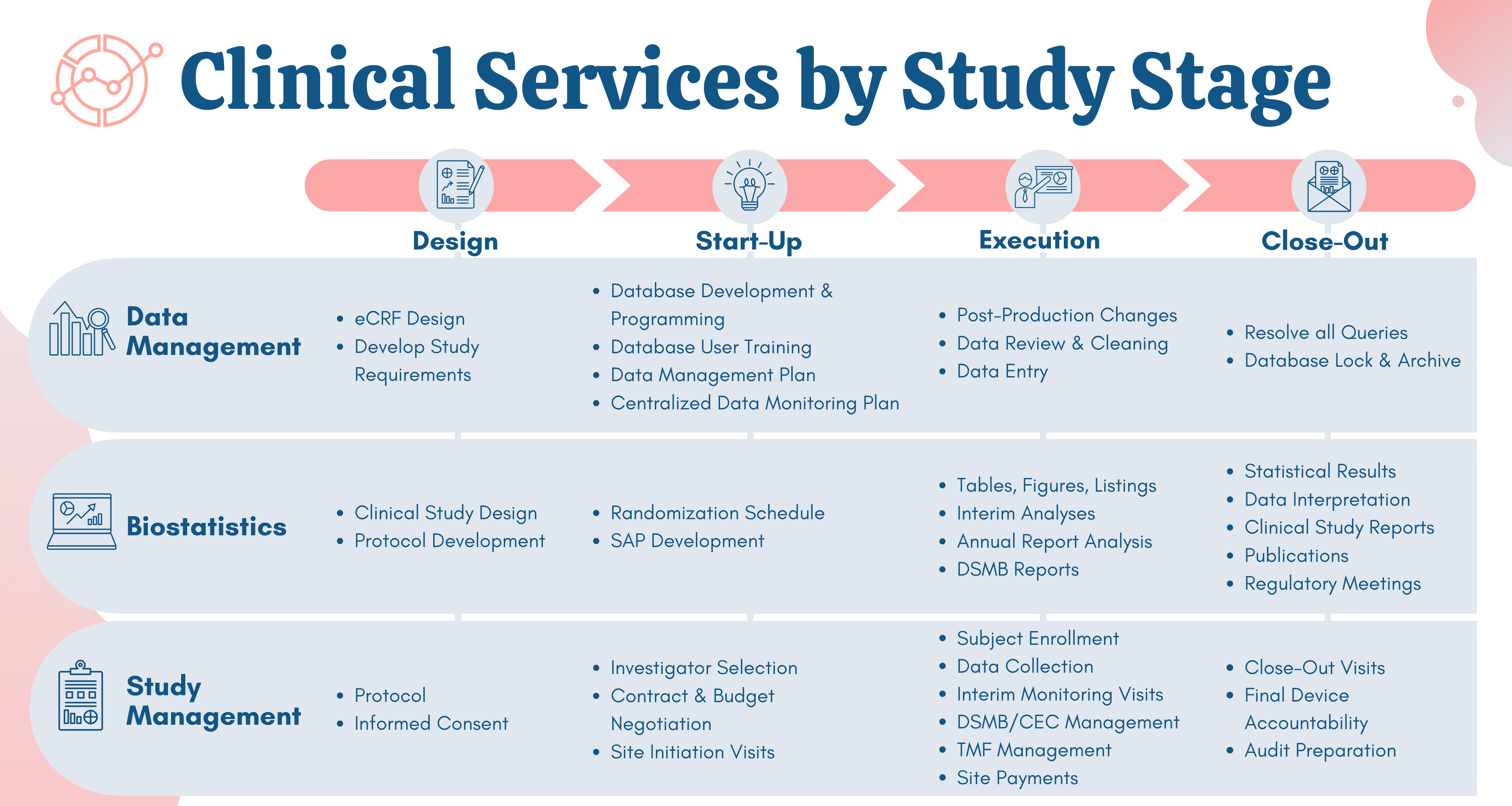
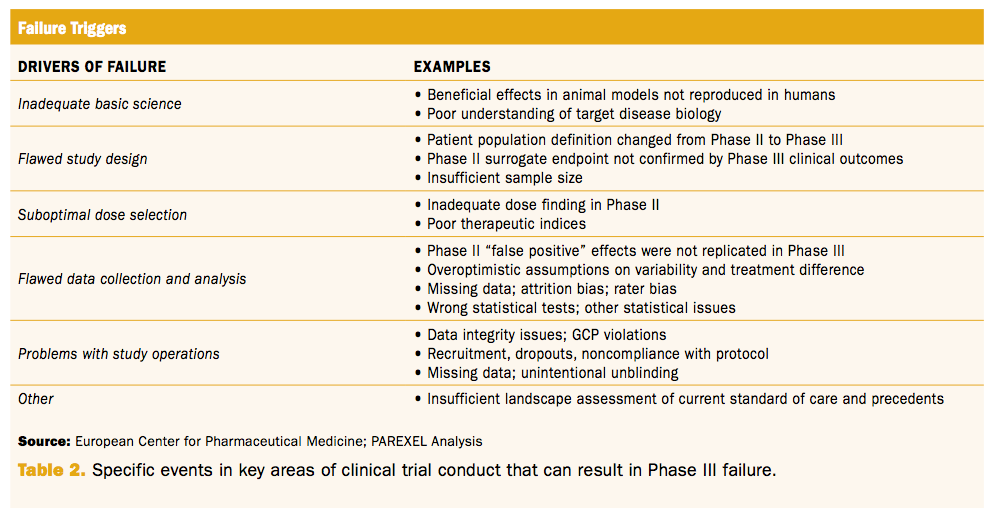
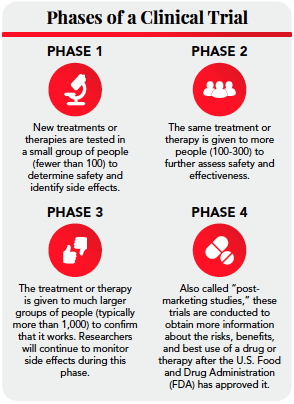
Clinical trial design and dissemination: comprehensive analysis of clinicaltrials.gov and PubMed data since 2005 | The BMJ
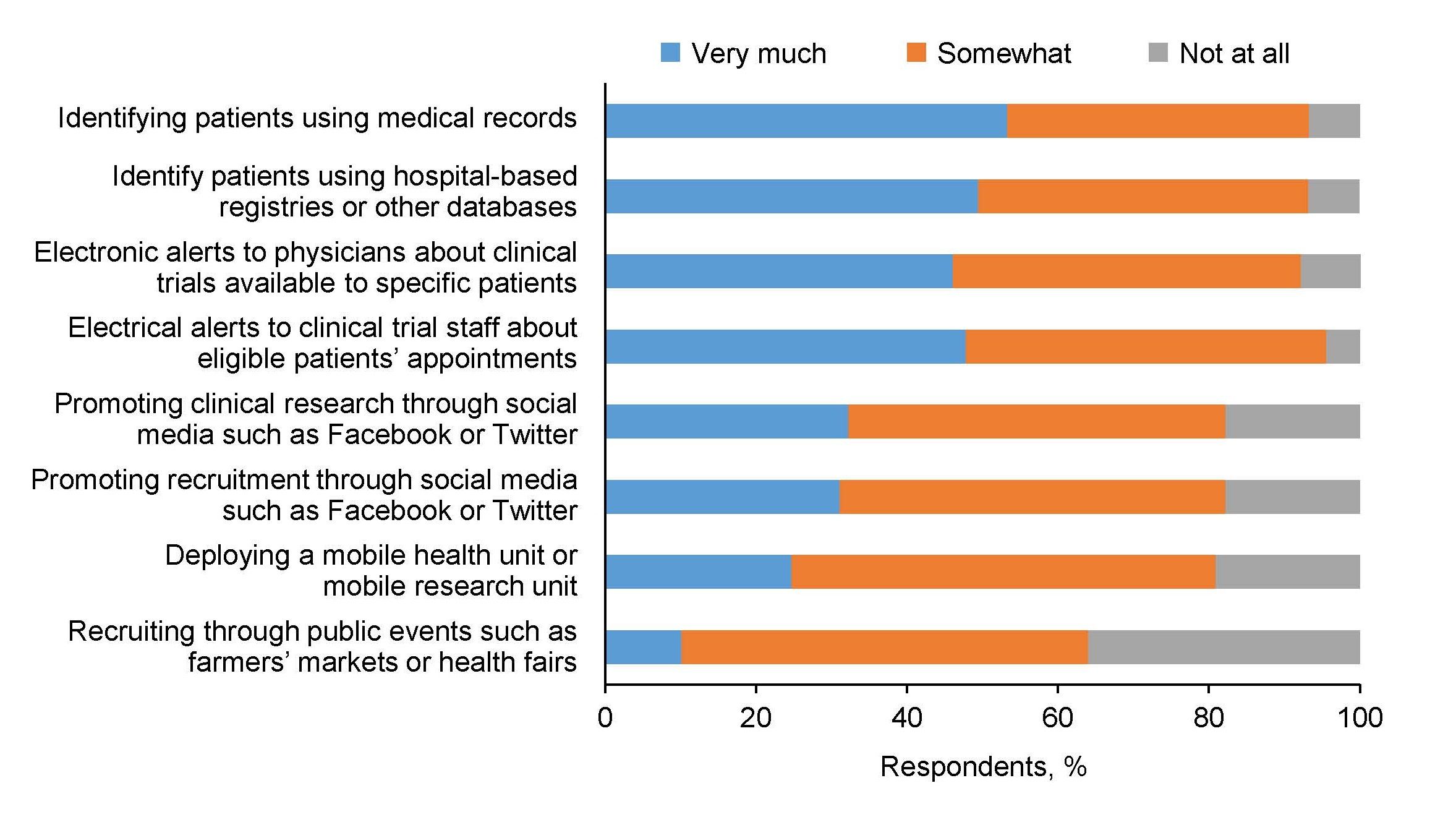
Frontiers | Factors Affecting Combination Trial Success (FACTS): Investigator Survey Results on Early-Phase Combination Trials

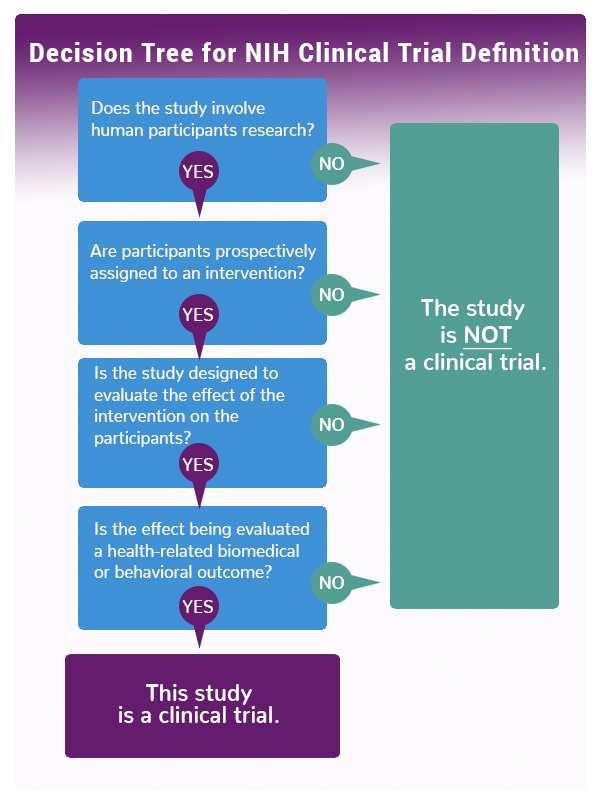
Compliance with legal requirement to report clinical trial results on ClinicalTrials.gov: a cohort study - The Lancet

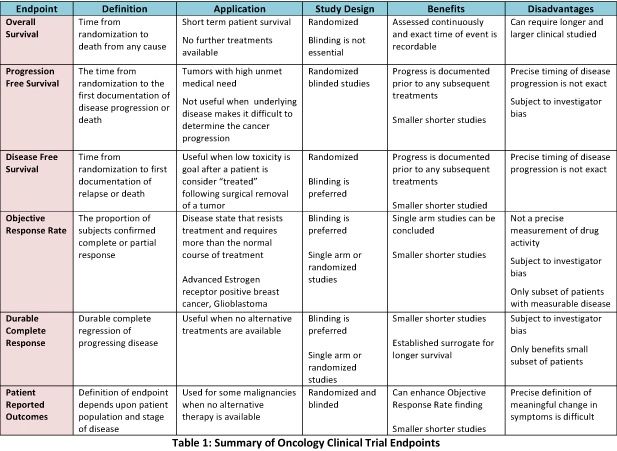
A Guide to Understanding Clinical Trials: Part 2 - Five Factors to Consider When Evaluating Results - Bench Press